Understanding Cellular Stress Response: The Role of Your Molecular Machinery
Tiny cellular machines like the eIF4F system help activate the body’s cellular stress response.
- Cells respond to stress, and usually, the eIF4E protein helps everything run smoothly. When eIF4E is low, a “fixer” protein (GCN4) helps with the cellular stress response.
- GCN4 is made in cells without needing eIF2a or intricate changes. It uses a special method where the cell’s protein-making machinery (ribosomes) works faster.
- A group of molecules called the ‘ternary complex’ builds proteins in cells. These stay the same even when eIF4E is missing, ensuring continual protein production.
- This discovery tells how stressed and nutrient-hungry cells manage protein, clearly showing cooperation between the two systems.
Study Uncovers How Cells React to Starvation
A research team at Washington University has found another way that cells deal with stress. Led by Kyusik Kim, the team saw that when a key molecule (eIF4E) gets used up, it sets a unique stress response in motion within the cell. This previously unknown process helps the cell survive by making a special protein (GCN4). GCN4 is needed to adapt your body during tough times. The fascinating part is that it occurs separately from the typical stress response. It is a big step towards understanding how cells control genes under stress.
Background and Methods Used
This research examined how cells build proteins using a team of molecules called the eIF4F complex. The main focus was on what happens when a key part (eIF4E) is missing. Scientists used specially made yeast cells with a weakened eIF4E. The idea behind their approach was to see how it affected protein building. To track the changes, the team used fancy techniques: ribosome profiling (watching the protein assembly line) and RNA sequencing (reading cell instructions). They carried out this experiment under normal conditions and when the eIF4E was weakened.
Cellular Stress Response: Key Findings
This table breaks down the takeaways from this trial. It shows what happens inside the cell’s protein-building machinery when a key molecule (eIF4E) is missing or in short supply.
| Key Findings | Details |
|---|---|
| TRANSLATION DYNAMICS | |
| Absence of eIF4E | Without the key molecule (eIF4E), the cell can still make a protein called GCN4. This tells researchers there’s another method besides the one they already knew about. |
| Depletion of eIF4E | When eIF4E runs low, it frees up another molecule (eIF4A). This helps ribosomes (protein builders) work faster and skip roadblocks (uORFs) that can otherwise slow things down. |
| MECHANISTIC INSIGHTS | |
| Regulation of GCN4 Translation | Making the protein GCN4 depends on a balance between two molecules (eIF4A and eIF4E) inside the cell. |
| Deletion of TIF1 | Without the helper molecule TIF1, cells can’t make GCN4 protein even when stressed, showing eIF4A is vital. |
How Cells Adapt During Tough Times
Cells are always building tiny machines called proteins, which are vital to keep things running well. This experiment focused on a protein named GCN4. The role of GCN4 is to help cells adapt when stressed or lacking nutrients.
Scientists now think that cells communicate between two critical pathways. These are the channels involved in protein building and stress response.
- Independent of the known eIF2a phosphorylation process.
- Shows a novel way that pathways talk to each other
- Hints at a new way these pathways might work together
Here is a bar chart illustrating GCN4 levels under various conditions:
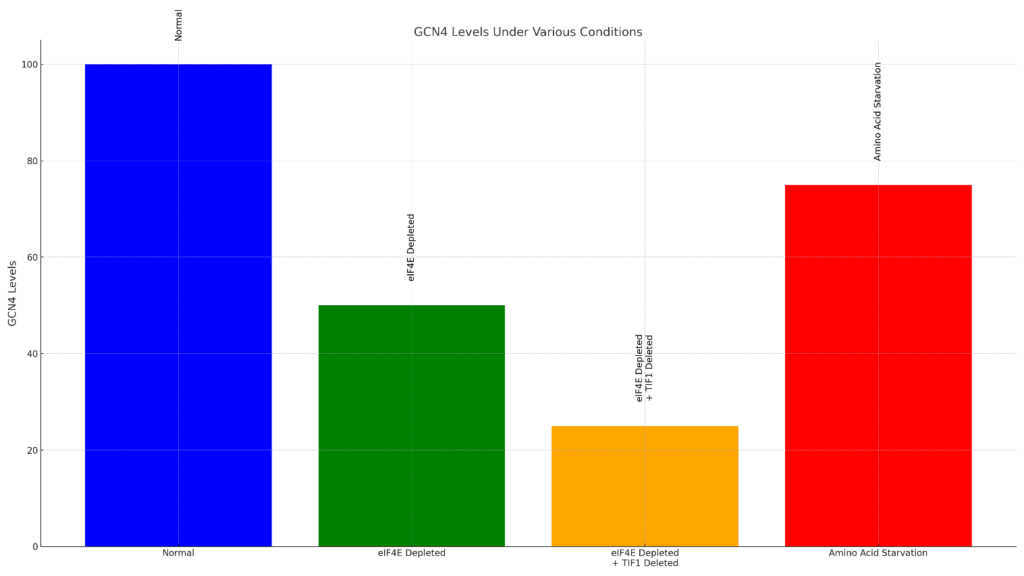
Normal: Everything is working as usual; all the needed parts are there.
eIF4E Low: A molecule important for protein building (like a factory worker) is in short supply.
Low eIF4E + No TIF1: Both the protein-building worker (eIF4E) is low, and another helper molecule (TIF1) is missing.
Cell Starving: The cell does not get the building blocks (amino acids) it needs to work.
New Clues About Cellular Stress Response
The trial revealed a surprise in how cells control their genes under stress. The group of proteins called the eIF4F complex plays a major role in this. The ways these components work together, depending on their relative amounts, surprised everyone. This knowledge could pave the way for new methods to manage stress responses in cells. Further studies will expand knowledge on the finer controls of genes in diverse situations.
Limitations and Future Directions
The study has taken scientific knowledge up a level, but there’s still more to learn. After all, this trial looked mostly at yeast cells. Future studies must see if these same ideas apply to more complex organisms like people. Scientists are also keen to know if controlling eIF4F molecules may be a way to treat diseases where stress responses are messed up. It’s an area that is definitely worth exploring further.
In Conclusion
This new study discovered ways that different parts of cells talk to each other when stressed or lacking nutrients. Normally, protein building and stress response work together in a particular way. Conversely, researchers saw that when a key molecule (eIF4E) is low, cells use a different method. That process involves another molecule (eIF4A) to keep making a protein called GCN4, which is needed for adapting to tough times. This shows us how smart cells are at adjusting their inner workings to survive. It gives experts a deeper appreciation of the way cells handle stress and may lead to new ways of treating diseases.
Resource links
Eif4f Complex Dynamics Are Important for the Activation of the Integrated Stress Response